Like many other countries, South Africa is struggling to access adequate testing materials to scale up the Covid-19 coronavirus testing.
A key challenge faced by laboratories in South Africa and elsewhere is that most of our diagnostic infrastructure requires the use of proprietary test materials – including reagents, consumables and cartridges.
This largely prevents laboratories from making their own or procuring test materials from sources other than the diagnostic machine’s manufacturer.
The National Health Laboratory Service (NHLS) is currently using diagnostic machines produced by several companies to diagnose Covid-19.
But the country is facing challenges in securing an adequate supply of reagents and consumables for running these tests.
The NHLS has also indicated that it will increase its use of the American company Cepheid’s GeneXpert machines for testing as test cartridges become available. Yet, despite being the first country to broadly roll-out GeneXpert diagnostic machines for TB testing, and having been a crucial location for their trialling, South Africa is struggling to access adequate Covid-19 test cartridges from Cepheid.
To address these problems, civil society organisations have called for greater transparency on the global distribution of test materials by diagnostic manufacturers. They have also called for a mechanism to pool and share knowledge and rights for health technologies that demonstrate efficacy in preventing, diagnosing and treating Covid-19.
Such a mechanism would allow other manufacturers to make and supply proprietary test materials and could also enable laboratories to prepare their own reagents when facing supply shortages.
Proprietary machines prevent countries from brewing their own reagents
In the early days of the Covid-19 outbreak, before commercial diagnostic tests were available and authorised for sale, the World Health Organisation (WHO) published a guideline of how laboratories could develop their own coronavirus diagnostic tests.
While our national laboratories can develop their own “home-brewed” or “in-house” reagents based on WHO guidelines, our testing infrastructure does not allow for the use of these reagents at scale.
This is because, like in many other countries, the vast majority of South Africa’s diagnostic machines are procured from commercial manufacturers whose machines require the use of their own proprietary test materials. The need for laboratories to manually prepare home-brewed reagents also limits their scalability.
Dr Marvin Hsiao, an NHLS virologist, says commercial diagnostic manufacturers develop their own tests, containing proprietary reagents and unique consumables and packing.
As a result, the tests cannot be interchanged between different diagnostic systems. Hsiao adds: “Even we don’t know what is in the proprietary reagents.”
This is because the specific formulations are protected as trade secrets. Hsiao says under normal circumstances, the use of proprietary commercial reagents is preferable to home-brewed reagents, as they are less labour intensive and allow for testing at scale.
However, the limitations of proprietary systems are becoming evident, as major international companies concede they cannot meet supply.
Swiss healthcare company Roche’s website, for example, states: “We are doing everything possible to produce as many tests as we can. Since this is a global health emergency, demand is outpacing supply.”
South Korean company Seegene, has notably scaled up test kit manufacturing to meet demand, but South Africa has less diagnostic capacity to process Seegene tests than Cepheid and Roche tests.
How is Covid-19 diagnosed?
Covid-19 is currently diagnosed using a diagnostic technique called polymerase chain reaction (PCR). PCR is commonly used to diagnose bacterial and viral infections, including TB, HIV, hepatitis B and C, human papillomavirus and others.
PCR tests are the most effective way to diagnose Covid-19 as they detect the virus and can therefore determine whether a person has an active coronavirus infection, unlike antibody tests which look at immune responses to determine if a person has ever been infected.
There are three critical stages involved in collecting and processing samples for the PCR diagnosis.
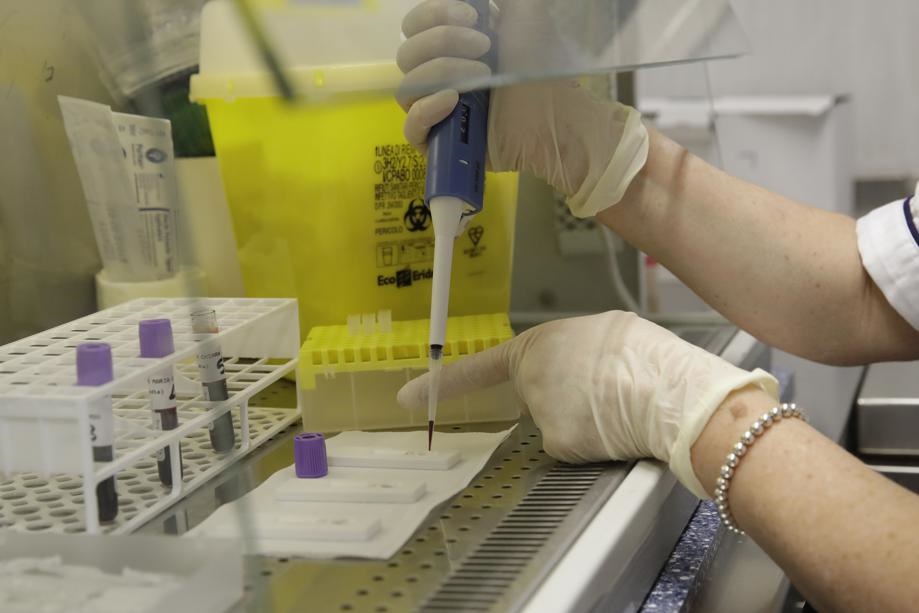
The first stage involves the collection of a sputum or phlegm sample from a patient’s nose or throat. This sample is then sent to a lab for processing in a sterile tube. The second stage involves the preparation of samples for testing (although this stage is unnecessary when using GeneXpert machines).
To prepare samples for testing, lab technicians must extract genetic material using an extraction reagent and consumables such as trays and pipettes.
The third and final stage involves the processing of the sample to detect Covid-19 genetic material using another specifically formulated reagent solution and a PCR diagnostic machine.
Reagents are liquid substances made from a blend of synthetic chemicals and enzymes.
While some shortages of raw chemicals needed for reagents have been reported, limitations on the manufacturing capacity of diagnostic manufacturers currently appears to be the main challenge in ramping up supply.
What diagnostic machines and tests is South Africa using for Covid-19?
South Africa is currently using Covid-19 test kits –which include reagents and consumables or cartridges – from the Swiss company Roche, the US companies Cepheid and Thermofisher and the South Korean company Seegene.
Roche and Cepheid tests can only be used on their own diagnostic machines. Seegene tests can be used on Seegene and Bio-Rad diagnostic machines, and Thermofisher tests can be used on Applied Biosystems QuantStudio and Bio-Rad diagnostic machines. Bio-Rad diagnostic machines can also be used to process home-brewed tests.
Using several tests and machines has allowed South Africa to avert testing interruptions due to shortages of reagents or cartridges for specific machines.
This has however also meant that lab technicians at the NHLS must regularly switch between diagnostic machines depending on what test materials are available.
Hsiao says that the need to constantly switch between diagnostic machines is impeding the efficiency of laboratory processes.
Where are the GeneXpert cartridges?
South Africa was the first country to roll-out GeneXpert diagnostics at scale after GeneXpert tests were shown to be effective in diagnosing TB. The country was also a crucial site for the trialling of the diagnostic machines.
The country first procured GeneXpert machines in 2011 – at about R600 000 per machine – excluding the cost of cartridges for processing tests. Today we have more than 300 GeneXpert diagnostic machines.
The NHLS has said it intends to use 180 of these machines – which can process between four and 80 tests simultaneously – for Covid-19 testing, but the slow delivery of test cartridges from Cepheid has thwarted the scaling up of tests.
According to Cepheid’s website, it began shipping Covid-19 test cartridges on March 25 2020.
NHLS chief executive officer Dr Kamy Chetty confirmed on April 16 that South Africa had received 10 000 cartridges and was expecting another 20 000.
The NHLS has not confirmed whether the additional cartridges have arrived, or how many have been received to date.
However, even with additional cartridges South Africa is still facing shortages.
Chetty says the country is sourcing enough cartridges from Cepheid to perform 10 000 tests per day.
It is unclear how the cartridge stock received compares to other countries, given a lack of transparency on global distribution.
However, there are concerns that developing countries are losing out to wealthier nations with deeper pockets and greater negotiating power in accessing test materials.
The Treatment Action Group’s (TAG’s) David Branigan says that besides affordable diagnostic pricing, “there is [an] urgent need for greater transparency and accountability of the criteria and mechanisms used by global health actors and by diagnostics companies for allocating available supply of tests”.
He says TAG, together with the Treatment Action Campaign and six other civil society groups, sent a letter on April 24 to the recently formed WHO-led Diagnostics Supply Consortium, calling on it to use its procurement and funding clout to negotiate affordable test prices based on cost-of-goods, and ensure equitable and transparent distribution of Covid-19 tests and materials.
The letter also called on the consortium to promote technology transfer to qualified manufactures to increase manufacturing and supply of diagnostic tests.
Technology transfer, trade secrets and patents
As major diagnostic manufacturers struggle to meet the demand for Covid-19 test materials, it may be necessary for governments and companies to enable the manufacturing of proprietary test materials by laboratories and competitors.
It is also conceivable that South Africa’s competition authorities, who previously played an important role in allowing more generic ARVs onto the market, may again be called on to intervene.
Besides sharing knowledge and pressuring companies to reveal access to inhibiting trade secrets, governments and the industry can use licensing, technology transfer and patent pooling mechanisms to enable the manufacturing of proprietary test materials by competitors and public manufacturers.
* This article was produced by Spotlight – health journalism in the public interest.




 Publications
Publications
 Partners
Partners